The Bhagi-Damodaran lab uses tools in chemistry and biology to discover new drugs and design novel catalysts. To achieve these goals, we focus on enzymes that use metals as cofactors, called metalloenzymes. Metalloenzymes are excellent candidates for drug discovery and catalysis as both biochemical (e.g. crystallography, activity assays, NMR) and inorganic chemistry (e.g. UV-Vis, EPR, EXAFS) techniques can be used to investigate and characterize them.
1. Targeting metalloenzymes involved in cellular signaling towards next-generation therapeutics
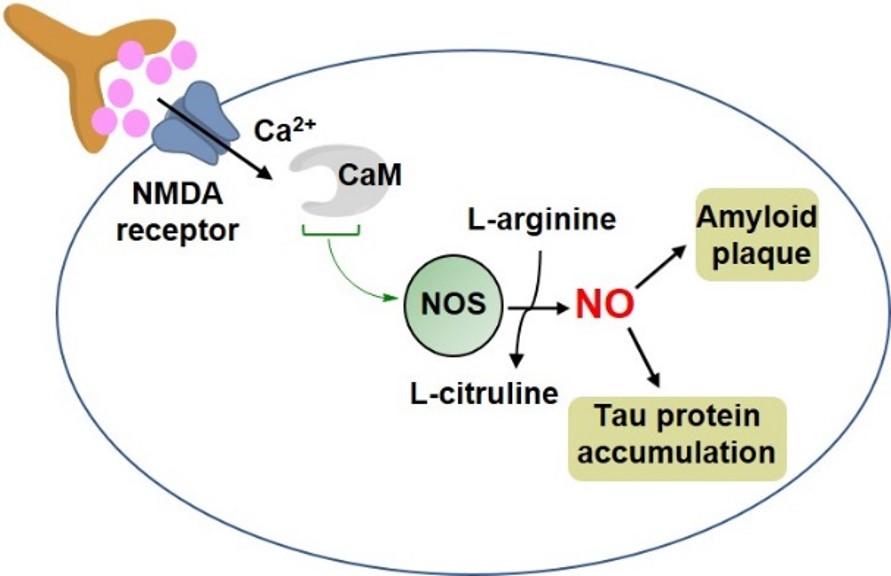
Despite comprising half of the proteome, metalloenzymes are targeted by only 4% drugs. We seek to change this paradigm in drug discovery methods. The overarching goal of the lab is to identify, characterize and target metalloenzyme-based redox signaling pathways towards the discovery of next-generation therapeutics. For instance, heme-containing metalloenzyme, nitric oxide synthase (NOS) is responsible for neuronal communication and is considered an important drug target for neurodegenerative diseases. Our lab seeks to elucidate structure-function relations in heme enzymes (like NOS in Fig. 1) to obtain a complete mechanistic understanding of metalloenzyme-based cellular signaling pathways. At the same time, we wish to utilize insights gathered from these fundamental studies to design novel drugs against diseases like Alzheimer’s and Tuberculosis. This work is highly multi-disciplinary and benefits from several on-campus research facilities like Molecular characterization facility, MNMR center, ITDD high-throughput screening core, Kahlert structural biology center, microbiology research facility and supercomputing center.
Drawing from core disciplines of chemical biology, inorganic chemistry, biophysics and organic synthesis, students/postdocs in Bhagi-Damodaran lab receive broad, fundamental training in experimental techniques ranging from small-molecule discovery to spectroscopy and electrochemistry to protein biochemistry to cell work.
2. Engineering new catalysts through rational design and directed evolution of metalloenzymes
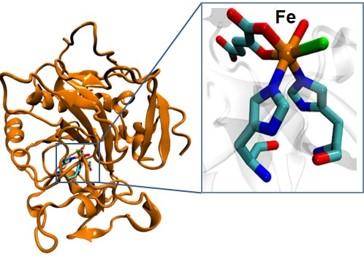
Metalloenzymes catalyze several challenging chemical reactions with high efficiency, using inexpensive precursors and at ambient conditions. For instance, heme iron containing P450 enzymes catalyze C-H bond oxidation, nonheme iron containing haolgenase enzymes catalyze C-H bond halogenation (Fig. 2). These enzymes, however, are highly substrate specific such that they are unable to catalyze a broad range of substrates from a chemical synthesis perspective. Our lab seeks to broaden the substrate scope of metalloenzymes (like nonheme iron halogenase) using computation-guided rational design and natural selection-guided directed evolution techniques. This research direction is highly interdisciplinary and benefits from collaborations with Larry Que, Jason Goodpaster, Mark Distefano and Will Pomerantz labs.